Chemistry Counting Atoms in Compounds Worksheet #7.0.1 delves into the fundamental principles of atomic composition and provides a systematic approach to counting atoms within chemical compounds. This engaging resource equips learners with a comprehensive understanding of the topic, fostering a deeper appreciation for the intricate world of chemistry.
Worksheet #7.0.1 serves as a valuable tool for students, guiding them through a step-by-step process of counting atoms using chemical formulas. The worksheet’s well-structured format and clear instructions enable learners to confidently navigate the concepts and apply them to various compound types.
Counting Atoms in Compounds
In chemistry, compounds are formed when two or more elements combine. The composition of a compound is determined by the number and types of atoms present in its molecules. Counting atoms in a compound is a fundamental skill in chemistry, as it provides insights into the structure and properties of the compound.
To count atoms in a compound, you need to know its chemical formula. A chemical formula is a symbolic representation of a compound that shows the elements present and their relative proportions.
Step-by-Step Procedure for Counting Atoms in a Compound
- Write down the chemical formula of the compound.
- Identify the elements present in the compound.
- For each element, count the number of atoms present in the formula.
- Multiply the number of atoms by the subscript of the element, if there is one.
- Add up the number of atoms for each element to get the total number of atoms in the compound.
Worksheet #7.0.1, Chemistry counting atoms in compounds worksheet #7.0.1
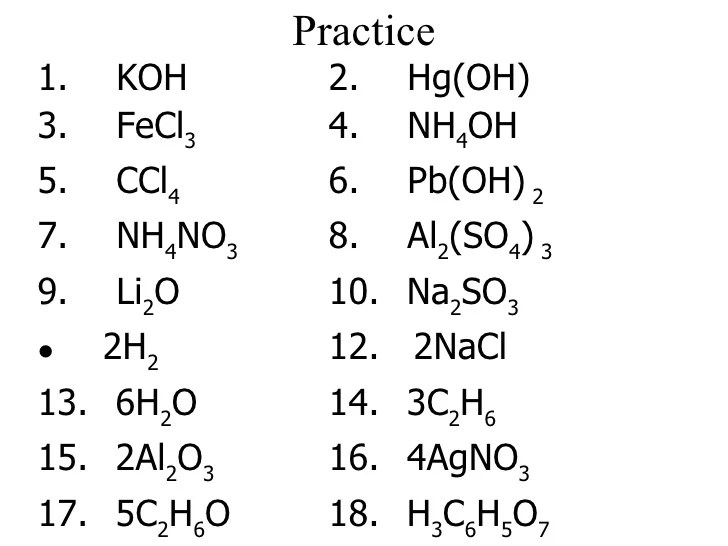
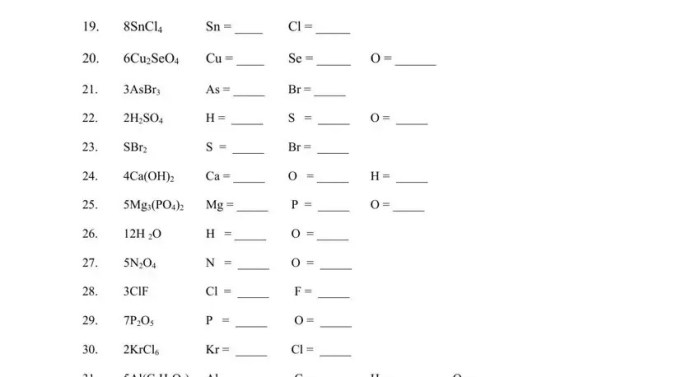
Worksheet #7.0.1 is designed to help students practice counting atoms in compounds. The worksheet contains a variety of problems that require students to count the number of atoms of each element in different compounds.
The worksheet is divided into two sections. The first section contains problems that involve simple compounds with no subscripts. The second section contains problems that involve compounds with subscripts.
Examples and Applications
- Ionic compounds:In ionic compounds, the atoms are held together by electrostatic forces between positively and negatively charged ions. For example, in sodium chloride (NaCl), there is one sodium atom for every chlorine atom.
- Covalent compounds:In covalent compounds, the atoms are held together by covalent bonds, which are formed when atoms share electrons. For example, in methane (CH 4), there is one carbon atom for every four hydrogen atoms.
Counting atoms is used in various chemical applications, such as:
- Determining empirical formulas
- Calculating molecular masses
- Predicting the properties of compounds
Methods and Techniques
There are two common methods for counting atoms in compounds:
- Criss-cross method:This method is used to count atoms in ionic compounds. It involves criss-crossing the charges of the ions to determine the number of atoms of each element in the compound.
- Oxidation number method:This method is used to count atoms in covalent compounds. It involves assigning oxidation numbers to the atoms in the compound and then using these oxidation numbers to determine the number of atoms of each element.
Each method has its own advantages and disadvantages. The criss-cross method is simpler to use, but it can only be used to count atoms in ionic compounds. The oxidation number method is more versatile, but it can be more difficult to use.
Extensions and Modifications
Worksheet #7.0.1 can be extended or modified to make it more challenging or tailored to specific learning objectives.
- Challenge problems:Add problems that involve more complex compounds or that require students to use both the criss-cross method and the oxidation number method.
- Real-world applications:Ask students to research how counting atoms is used in different chemical applications, such as determining empirical formulas or calculating molecular masses.
Additional activities or resources that can complement the worksheet include:
- Interactive simulations:Use interactive simulations to allow students to explore the structure of compounds and count atoms in a virtual environment.
- Online quizzes:Create online quizzes to assess students’ understanding of counting atoms in compounds.
Visual Aids and Illustrations
| Compound | Chemical Formula | Number of Atoms |
|---|---|---|
| Sodium chloride | NaCl | 1 sodium atom, 1 chlorine atom |
| Methane | CH4 | 1 carbon atom, 4 hydrogen atoms |
| Water | H2O | 2 hydrogen atoms, 1 oxygen atom |
Flowchart for Solving Problems on Worksheet #7.0.1
- Start
- Write down the chemical formula of the compound.
- Identify the elements present in the compound.
- For each element, count the number of atoms present in the formula.
- Multiply the number of atoms by the subscript of the element, if there is one.
- Add up the number of atoms for each element to get the total number of atoms in the compound.
- End
Q&A: Chemistry Counting Atoms In Compounds Worksheet #7.0.1
What is the purpose of Worksheet #7.0.1?
Worksheet #7.0.1 provides a structured approach to counting atoms in chemical compounds, enhancing students’ understanding of atomic composition.
How does the worksheet benefit students?
The worksheet guides students through a step-by-step process, enabling them to confidently count atoms in various compound types.
What are the applications of counting atoms?
Counting atoms is crucial for determining empirical formulas, calculating molecular masses, and understanding chemical reactions.