Which statement is true about metallic bonds – Metallic bonds, the cornerstone of metallic materials, are a fascinating subject that has captivated scientists and engineers for centuries. This article delves into the intriguing world of metallic bonds, exploring their formation, properties, and applications.
Metallic bonds arise from the unique behavior of valence electrons in metal atoms. These electrons are loosely bound to their respective atoms, forming a “sea” of mobile electrons that permeate the entire metal lattice. This electron sea model underpins the remarkable properties of metals, including their exceptional electrical and thermal conductivity, malleability, ductility, and characteristic luster.
Metallic Bond Characteristics
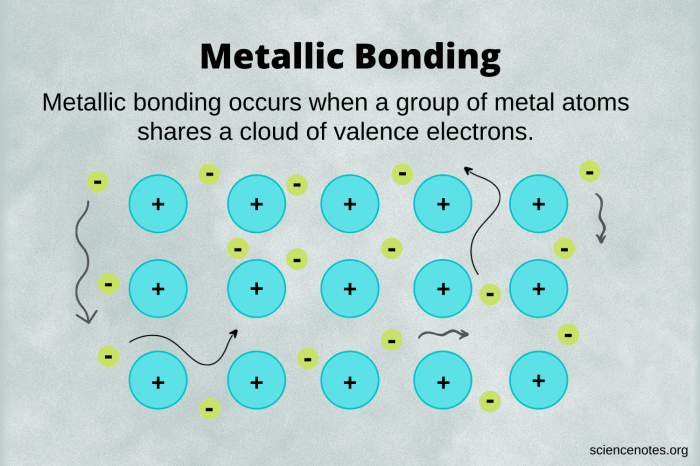
Metallic bonds are the forces that hold metal atoms together in a metallic lattice. They form when metal atoms lose their valence electrons, creating a sea of mobile electrons that surrounds the positively charged metal ions.
The electron sea model describes the metallic bond as a sea of valence electrons that are delocalized throughout the metal lattice. These electrons are not associated with any particular atom but are free to move throughout the metal. The positive metal ions are embedded in this sea of electrons, and the electrostatic attraction between the ions and the electrons holds the metal together.
Metallic bonds are stronger than ionic bonds but weaker than covalent bonds. This is because the electrostatic attraction between the positive metal ions and the negative electrons is weaker than the electrostatic attraction between the positive and negative ions in an ionic bond, but stronger than the covalent bond formed by the sharing of electrons between two atoms.
Properties of Metallic Bonds
The properties of metallic bonds give metals their characteristic properties.
- High electrical and thermal conductivity:The mobile electrons in a metallic bond can move freely throughout the metal lattice, which allows metals to conduct electricity and heat very well.
- Malleability and ductility:The mobile electrons in a metallic bond allow metal atoms to slide past each other easily, which makes metals malleable and ductile.
- Luster and reflectivity:The mobile electrons in a metallic bond can reflect light, which gives metals their characteristic luster and reflectivity.
Applications of Metallic Bonds
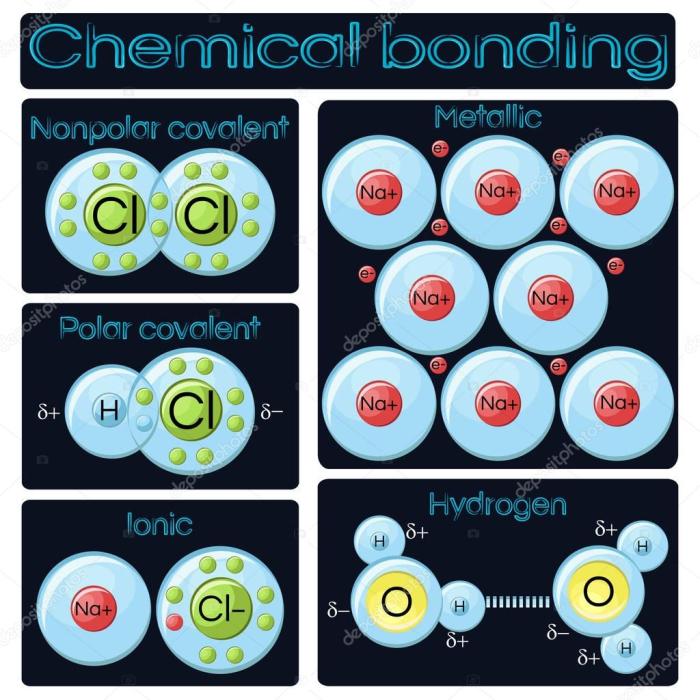
Metallic bonds are essential for the properties of metals, which make them useful in a wide variety of applications.
- Common metals and their applications:Metals such as iron, aluminum, copper, and gold are used in a wide variety of applications, including construction, transportation, and electronics.
- Alloys and composites:Alloys are mixtures of two or more metals, and composites are mixtures of metals with other materials such as ceramics or polymers. Alloys and composites can have properties that are different from the pure metals, and they are often used in applications where specific properties are required.
- New materials:Metallic bonds are being used to develop new materials with unique properties. For example, metallic glasses are amorphous metals that have the strength of metals but the flexibility of glasses.
Comparison of Metallic and Non-Metallic Bonds
| Characteristic | Metallic Bond | Non-Metallic Bond |
|---|---|---|
| Electronegativity difference | Low | High |
| Bond strength | Strong | Weak |
| Electrical conductivity | High | Low |
| Thermal conductivity | High | Low |
| Malleability | High | Low |
| Ductility | High | Low |
| Luster | High | Low |
User Queries: Which Statement Is True About Metallic Bonds
What is the electron sea model?
The electron sea model describes the behavior of valence electrons in metals. These electrons are not bound to specific atoms but instead form a mobile “sea” that permeates the entire metal lattice.
Why are metals good conductors of electricity?
The mobile valence electrons in metals allow for the easy flow of electric current, making metals excellent conductors of electricity.
What gives metals their characteristic luster?
The electron sea model also explains the luster of metals. When light strikes a metal surface, the mobile electrons absorb and re-emit the energy, giving metals their shiny appearance.